From business to the use case
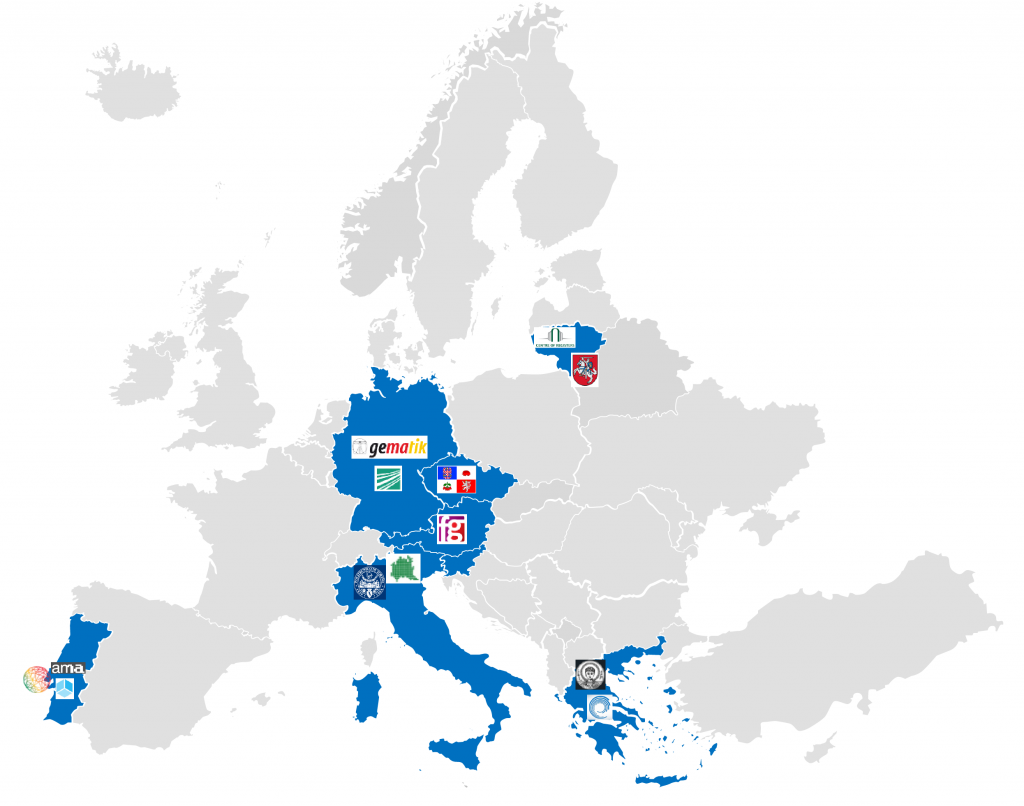
The consortium is composed by the competent organisations of 7 MS responsible for the eIDAS node and the NCPeH from each country. Where necessary these organizations brought in additional national organization (i.e. Greece, Portugal) or mandated other national organizations to carry out the tasks on their behalf (i.e. Italy, Czech Republic).
- Portugal
SPMS (Shared Services for Ministry of Health)
AMA (Administrative Modernization Agency)
Caixa Mágica Software (CMS)
- Austria
ATNA (Ministry of Health)
- Italy
POLITO (Politecnico di Torino)
LISPA (Lombardia Informatica S.p.A.)
- Germany
Gematik
- Lithuania
State Enterprise Centre of Registers
IT and Communications Department
- Czech Republic
Vysočina
- Greece
Helenic Ministry of Administrative Reconstruction (HMAR)
IDIKA S.A
AUTH – The Information Technology Laboratory of the Aristotelian University
TRANSFERATHON 28th – 30th October 2019

Representatives from 11 Member States, DG SANTE, DG DIGIT and eHMSEG travelled to Porto to attend TRANSFERATHON, currently taking place at the SPMS, EPE facilities.
Through the three-day event, HEALTHeID Consortium will be working together with Member States participants, as well as from the Commission to demonstrate the practical use of the HEALTHeID NCPeH-eIDAS connector, focusing on the validation of eID in the Health domain.
With two parallel working sessions: Business Workstream and Technical Workstream, TRANSFERATHON includes a set of workshops and hands-on demonstrations.
HEALTHeID – TRANSFERATHON main purpose is supporting European countries in integrating eIDAS (Electronic IDentification, Authentication and Trust Services) with cross-border services in order to improve the quality of services provided to citizens in Europe.
TRANSFERATHON |HEALTHeID Project DEMO

TRANSFERATHON will take place at the SPMS, EPE facilities in Porto, from 28th to 30th October 2019.
Within the Project HEALTHeID – aimed at integrating Electronic Identification, Authentication and Trust Services (eIDAS) with cross-border services, to improve health services provided to EU citizens, TRANSFERATHON comprises a set of technical workshops and practical demonstrations.
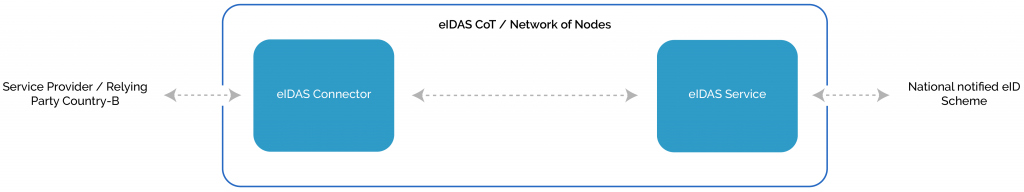
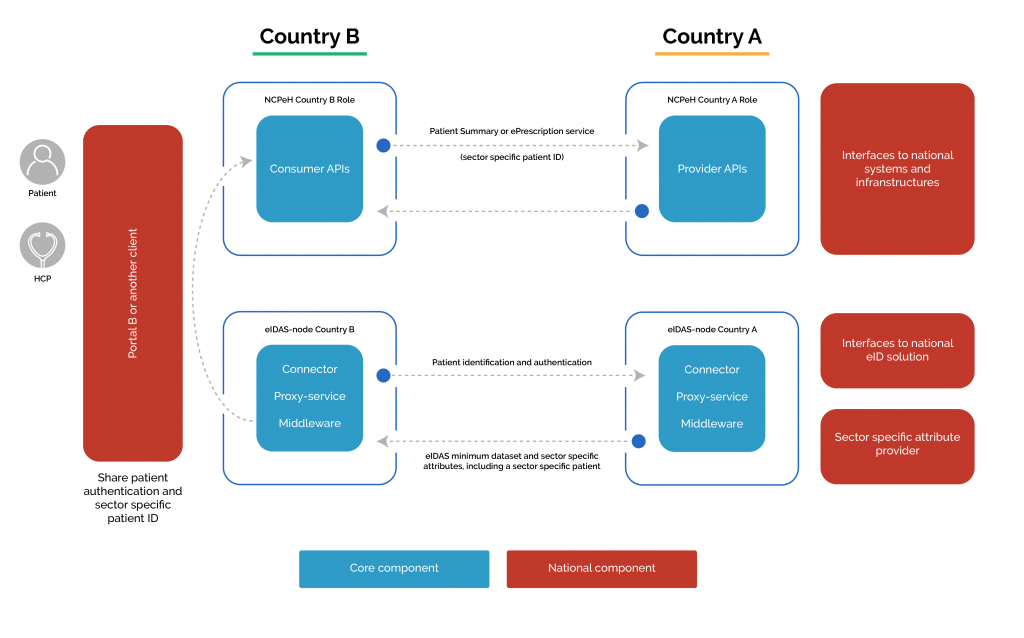
As the name suggests, TRANSFERATHON strives for the transferability of the reference implementation to all Member States. To this end, practical demonstrations of the use of the connector developed for the eHealth domain will be performed, focusing the validation of electronic identification (eID) in health domain by connecting eIDAS node with National Contact Points for eHealth (NCPeH) environments.
Representatives from DG SANTE, DG CONNECT, DG DIGIT, eHMSEG Chairs, Member States, entities responsible for eIDAS node & for NCPeH, National authorities, Competence Centres and Academia are the expected attendees for this three-day event.
Enabling citizens’ access to digital health records and services in Europe, ensuring digital transactions security are HEALTHeID main goals, hopefully TRANSFERATHON may be the kick-off.
4th HEALTHeID – Technical Working Meeting

The 4th HEALTHeID – Technical Working Meeting took place in Prague, Czech Republic, July 16th – 17th this year. Olšanka Hotel welcomed the working group focused on project activities’ development during the two-day meeting.
Diogo Martins – International Projects Coordinator from SPMS introduced both sessions, followed by the work state of play and discussion of ideas.
The first day’s agenda focused on the progress of HEALTHeID Activity 2 “Development and testing of the reference implementation”, which aims to identify functional specifications for the eID component, develop and test its reference implementation, based on e-SENS eHealth eID software. This work, carried out by a highly specialized team, aims to collaborate with the experts leading the technical implementation at national level in the main Member States to ensure appropriate technical alignment at national level. In this sense, the working group also focused on the involvement of these entities, foreseeing their participation and contribution.
On the second day Activity 1 of the Project “Identification of non-functional requirements” marked the beginning of the meeting, which consists of identifying non-functional requirements, relating political, organizational and legal aspects, as well usage guidelines for the components of eID solution, resulting from the action.
As electronic identification is an essential mechanism to ensure digital transactions’ security, especially regarding health data, in the framework of Activity 3 “Preparation for transfer of activities” the action plan, transfer processes and methodology were then discussed.
Objectively, HEALTHeID implementation envisages the identification and cross-border authentication of citizens in the health field, enabling digital access to health records and prescription services in Europe.
HEALTHeID Session at Lisbon eHealth Summer Week 19th-22nd June 2018

“Nobody else is building HEALTHeID. That’s us!” were the words of Henrique Martins – Chairman of the Board of SPMS during the Project’s first workshop, on the second day of the 2nd Lisbon eHealth Summer Week. The event took place at Belém Cultural Center aimed for eHealth-focused project meetings, fostering discussion of development strategies, implementation, added value and the future of different eHealth services that are critical to health.
Carried out by a consortium of European countries HEALTHeID foresees the identification and cross-border authentication of citizens in the health field, allowing the access to digital health records and electronic prescription services in Europe. Electronic identification is a key mechanism to ensure the security of digital transactions, especially when it comes to health data.
At this first meeting, implementation and its challenges were a priority, focusing on raising technical and legal issues such as the adoption of eIDAS (Electronic IDentification, Authentication and trust Services) regulation.
As HEALTHeID Coordinator, SPMS will continue to hold meetings for the Project development, seeking to improve the health system for citizens and professionals across Europe.
Transferathon
28th – 30th October
Agenda